Abstract
Regulatory T-cells (Treg) play a critical role in preventing autoimmune and alloimmune reactions. Clinical trials demonstrated that Treg adoptive transfer significantly reduced but did not eliminate grades II-IV graft-vs-host disease (GVHD).
We hypothesized that increasing fatty acid oxidation (FAO) would augment Treg potency, and ultimately enhance Treg-mediated GVHD reduction. Acetyl-CoA carboxylase-1 (ACC1) negatively regulates FAO by augmenting fatty acid synthesis (FAS), which results in downstream inhibition of CPT1a, the rate-limiting enzyme in FAO. ACC1 genetic deletion or inhibition increases T-cell FAO and skews naive CD4 T-cell differentiation from Th17 to Treg. However, the role of ACC1 in thymic Treg function and metabolic fitness is unknown.
Here we show that a 2-hour treatment of murine Treg with ND630, a pharmacological inhibitor of ACC1 currently in clinical trials for hepatic steatosis, enhances Treg suppressive function. As compared to control Tregs, ND630 treatment also increased expression of suppressive molecules Lag3, TIGIT, and IL-10. Treatment of Tregs with either of two other ACC1 inhibitors (TOFA, sorafin A) resulted in similar augmentations in Treg function, disfavoring the likelihood of off-target effects. Additionally, ND630 treatment increased Treg oxygen consumption rate (OCR), a surrogate for mitochondrial oxidative phosphorylation (OXPHOS), a critical source of ATP for Treg. Similar to murine Treg, ND630 treatment of in vitro expanded human Treg enhanced their suppressive function and OCR/OXPHOS. IACS, an OXPHOS inhibitor, abrogated the positive ND630 effect on in vitro Treg suppressive function and OXPHOS.
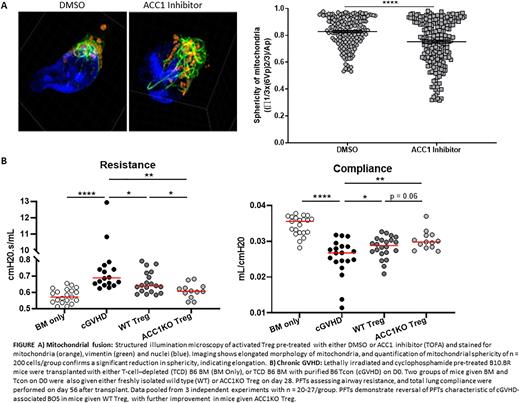
Given the augmentation in OXPHOS with ND630 treatment, we further evaluated ND630 effects on Treg mitochondria. Compared to controls, ND630 treatment increased mitochondrial mass and membrane potential, essential for energy storage during OXPHOS, as well as mitochondrial polarization, which is required for full Treg functionality. Microscopy analysis revealed enhanced mitochondrial fusion and elongation after ND630 or TOFA treatment (FigA). This mitochondrial structural change boosts OXPHOS, consistent with a direct effect of ACC1 inhibition on mitochondrial fitness.
Since ACC1-driven FAS ultimately inhibits FAO, we next evaluated contributors to FAO. Expression of CPT1a, a critical enzyme for mitochondrial FAO, fatty acid binding protein 5, a key shuttle of fatty acids (FA), and exogenous FA uptake were all enhanced after ND630 treatment. Using an unbiased metabolomic screen, a metabolic signature was observed that was consistent with enhanced FAO. Together, these data suggest that ND630 increases mitochondrial fitness, and augments both FA uptake and FAO.
To identify additional ND603-induced intracellular effects in Treg, activated murine Treg were subjected to RNAseq. Differential gene expression analysis indicated that 82 genes differed between DMSO and ND630 treated Treg. These included increased expression of genes associated with OXPHOS, as well as mTOR, consistent with high OXPHOS and suppressor cell function. Flow cytometry analysis of phosphorylated proteins showed reduced pFOXO3a and pAKT, both mTORC2 targets, but increased mTORC1 downstream phospho-proteins. An imbalance of mTORC1/2 is consistent to our previous studies reflective of higher Treg function and metabolic fitness (McDonald-Hyman C, JCI 2018).
To exclude potential bystander effects of ND630 on Treg function, we utilized murine Treg with genetic deletion of ACC1 (ACC1KO). Supporting our studies with chemical ACC1 inhibitors, ACC1KO Treg were functionally and metabolically superior to wild-type Treg. Furthermore, as our goal is to use ACC1 inhibited or deleted Treg in treatment of GVHD, we tested ACC1KO Treg in treating established bronchiolitis obliterans (BOS) in a murine model of chronic GVHD. ACC1KO Treg were more potent than wild-type Treg in reversing BOS pathology (FigB), a finding consistent in each of 3 replicate transplants.
In summary, ACC1 inhibition or deletion amplifies murine (and human) Treg potency resulting in superior metabolic fitness, and greater efficacy in treating established BOS in murine chronic GVHD. Since ND630 is already under investigation in patients, ACC1 inhibition with ND630 could be readily translatable for clinical trials with a goal of utilizing ACC1 inhibited Treg infusion to improve GVHD therapy.
Disclosures
Blazar:BlueRock Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rheos Medicines: Research Funding; Tmunity Therapeutics: Current holder of stock options in a privately-held company; Magenta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Carisma Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal